25 Kasım 2015 Çarşamba
WGCNA -- Another way to post-process your quantitative data
I think its about time we start some concerted spy missions. The genomics people have all sorts of cool tools. Lets send proteomics people to genomics meetings and then just steal all their cool ideas, which is probably less like stealing because they give away virtually all of their algorithms.
Case in point? This WGCNA thing. Notice that its been around since 2008. So..around the time we were all getting our heads wrapped around target decoy searches, the genomics people were like "hey, lets do some unsupervised clustering of our thousands of quantitative changes and see what stands out in a hierarchical sense"
What's it do? It tries to find patterns in your complex data without you spending all day looking up genes that make sense. Its just pulling out common traits and clusters them with your sample(s) and type(s).
How's it do it? Well, its in R, so it does it in the ugliest way possible. Does it look like the screen of a Commodore 64? Yup! Then its likely R, he world's most powerful and utilized statistical software package!
Besides that? I have no idea. Google Scholar informs me that the initial paper describing this algorithm has been cited nearly 1,000 times. So, somebody has liked this paper. Maybe I'd even toy with it before trying to check the Stats.
You can visit the WGCNA website here. It has links to papers and full tutorials.
Shoutout to Alexis for mentioning this algorithm to me a second time and showing me cool clustering data from it so I'd remember to share with people!
Ready to get HYPE(d) and AMP(ed)?
At first you might think I was initially a fan of this paper because looking up the protein and nucleotide in the title is a gold mine on Google images. I'm not saying you're wrong, but there is more to this cool paper even than the JPGs I'm probably going to insert while I'm writing this.
A few years ago people were pretty hyped about AMP. Remember this?
It looked like AMP was going to be a great big important PTM. Turns out, however, that its an absolute pain in the foot(?) to study with LC-MS. A couple labs gave it a good hard try and got some nice results, but their techniques maybe looked a little too painful for us to want to replicate.
In this new study in MCP from Malgorzata Broncel et al., we get to see a high throughput, high sensitivity, high resolution way of studying this modification!
How'd they do it? Without radiation! They used inert chemical probes that are attached to analogues of AMP. This technology was developed by other groups for doing visualization or protein arrays. Turns out, though, that they make a nice target for selectively pulling down proteins that had picked up the labeled AMP, and are conducive to ionizing and fragmenting in a Q Exactive.
If you are interested in this PTM, you've got a protocol now! And along the way they studied some super important protein named HYPE that has a lot to do with bacterial infections and may lead to nice drug targets for us to use in the post-antibiotic era!
24 Kasım 2015 Salı
Single protein equilibrium in a cell
Wow. This is just fascinating! Thanks PastelBio for sharing.
This link will take you to what I'm talking about. Its a press release of sorts about a new study in Nature Physics. Interestingly, from the time of the press release until now, it appears that they decided to change the name of the paper and it is available here (paywalled, sorry!)
The press release explains it better than I can, honestly, but they took that crazy super computer down in Tennessee, the Titan (19,000 CPUs AND 19,000 GPUs)
What they did was single cell protein modeling with all that processing power.
What did they conclude? That considering protein production speed and turnover via degradation, proteins may never actually achieve equilibrium within a cell. That you'd constantly be looking at a moving and changing population of even one single gene product. So if you were able to pull out just two proteoforms of a given gene product at any point in time, they probably wouldn't be the same. One might be just formed and the other might be partially degraded...or reacted, or modified.
Is it real? Who knows? Its fascinating, though! I guess it probably doesn't affect what we do much. I mean, we're looking at the averages of signals from thousands of copies of proteins from thousands or millions of cells at once, so maybe all of this averages out into just noise, but I always love things that highlight how little we still know about biology.
This link will take you to what I'm talking about. Its a press release of sorts about a new study in Nature Physics. Interestingly, from the time of the press release until now, it appears that they decided to change the name of the paper and it is available here (paywalled, sorry!)
The press release explains it better than I can, honestly, but they took that crazy super computer down in Tennessee, the Titan (19,000 CPUs AND 19,000 GPUs)
What they did was single cell protein modeling with all that processing power.
What did they conclude? That considering protein production speed and turnover via degradation, proteins may never actually achieve equilibrium within a cell. That you'd constantly be looking at a moving and changing population of even one single gene product. So if you were able to pull out just two proteoforms of a given gene product at any point in time, they probably wouldn't be the same. One might be just formed and the other might be partially degraded...or reacted, or modified.
Is it real? Who knows? Its fascinating, though! I guess it probably doesn't affect what we do much. I mean, we're looking at the averages of signals from thousands of copies of proteins from thousands or millions of cells at once, so maybe all of this averages out into just noise, but I always love things that highlight how little we still know about biology.
22 Kasım 2015 Pazar
Can you run Proteome Discoverer on Windows 10?
I've been asked this question a couple of times. And maybe now I know the answer.
At first run, it certainly looks like PD 2.1 installs just fine with nothing special whatsoever on Windows 10.
Now...that being said, this isn't officially supported by the vendor. And just because the couple HeLa runs I just did seem to go just fine doesn't mean that every feature will be ready...but, again, I haven't had a problem yet!
19 Kasım 2015 Perşembe
Nice and short review on proteogenomics in Springer
Like an awful lot of people right now, I'm kind of obsessed with the magical thing called "proteogenomics". Which...honestly...seems a little bit like magic. Getting good quality transcriptional data and filtering it so that you can see new mutations....that you can trust...AND THEN using this information to find new matches to your MS/MS data....that you can trust....
A few people have totally pulled it off....and I have the papers stacked up in front of my PC all marked up and highlighted and...well...maybe I'm dumb...but I still don't know how they did it.
For a good starting point, check out this nice review from Sam Faulkner et al.,. While I'm on the topic of magic, you might be surprised to see this is an article from Springer that isn't behind a paywall!
Oh yeah! And here is the link!
18 Kasım 2015 Çarşamba
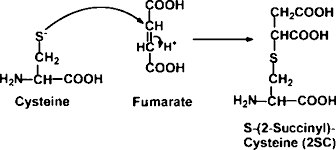
2SC --- another important PTM to look for?
Great...another protein post-translational modification...cause my search space isn't nutso enough already....
Oh! Hi, there! Ever looked at this one? Its called 2SC...and it turns out that it has a role in human diseases. Its even detectable by other molecular techniques and has been implicated in diabetes. A brand new paper from Gianluca Miglia et al., takes a computational approach and analyzes a ton of proteins. Turns out that it mostly sticks to Cysteines. Which makes me wonder if our typical techniques of cysteine reduction/alkylation/assumption of 100% cysteine alkylation means that we probably don't see it in our RAW data and can't see it in our processed data if it was there.
The abstract suggests that this modification is prevalent enough that they have seen multiple 2SC modifications per individual protein. I can't get much further because I'm stuck behind a paywall at this Panera this morning. An Open Access paper on this modification in people is available here, though its worth noting that they appear to have done all their work with Western blots. As a side note, it appears there are a lot of journals out there, and it appears that people are running out of names for them.
Ever wanted a great label free proteomics dataset?
"Hey guys, here's this awesome new algorithm for label free analysis!!!!"
Want a GREAT dataset to test it out on?
Check out this cool new paper from Claire Ramus et al.,! In this, they developed a great standard that all of us can download and use for free. They also use it to test a bunch of the current algorithms people are using.
The sample is the Sigma UPS1 48 protein mix (all equimolar proteins) spiked into a Yeast digest background at different concentrations. The spike-ins range from 50amol to 12,500 amol (yeah...there is a more efficient way of writing this, but I'm not great at metrics...thanks American public schools! I love sounding like an idiot to everyone else in the world...)
You can download the dataset...wow...my internet is slow right now...well, I guess you can download the dataset...from PRIDE. It will be PXD0001819 after it is published. The authors were kind enough to provide a way to download the dataset prior to publication (wow, right!??!) that you can find in the abstract.
Got a couple files. This study was on an Orbitrap Velos running High-Low (FT for MS1 and CID ion trap MS/MS scans). It looks like 60k resolution at the MS1 (around 400m/z)
Kaydol:
Yorumlar (Atom)
Popular Posts
-
A recent paper in PNAS makes the statement in the title "Protein Carbamylation is a Hallmark of Aging. You can find it here . They find...