16 Ekim 2015 Cuma
Amino acid composition and effects of mutations
That's pretty clear, isn't it? Just kidding, I have NO idea what that is, but its a figure from this paper, that does actually make a lot of sense.
Here is the gist of it: mutations are gonna happen. If they didn't evolution, to a large degree, wouldn't happen either (hugely complicated higher organism silly reproductive procedures aside). What Sahand Hormoz is proposing here is that the evolutionary pressure on essential proteins and their structures is to minimize the nasty effects of mutations is a mathematical certainty. That's a long sentence and I think the grammar could use some work.
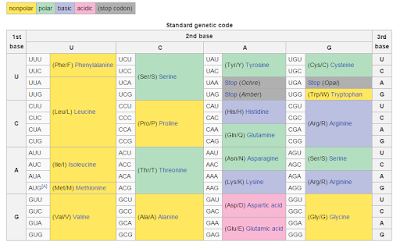
Here, I've stolen the genetic coding table from this Wikipedia page. Say, you've got an essential leucine at this essential location. That's great! Cause many point mutations can happen to the section of the DNA and the protein won't change at all! You can change UUA to UUG or CUA and you're still gonna have leucine! Now, if it goes UAA...well you've got a truncated protein...so maybe this wasn't the best example.
There are also changes where we switch amino acids and it isn't all that bad either. For example, it would be better to have a mutation that changes a polar amino acid with a small functional group to another similar one, but changing to one with a huge non-polar, would be a whole lot worse.
From the genetic perspective, this makes all sorts of sense. What this guy did was show that you can calculate it with that Maths stuff to show that this wasn't accidental at all. This was evolutionary pressure over billions of years to find the best possible way to protect the proteins that life would end without. Really cool read, even if you don't know what any of the equations mean!
Hey, Maryland and D.C (and surrounding areas!) Wanna come to the NIH for this awesome workshop?
Hey! I know this is self-serving, but enrollment for the NIH Proteome Discoverer 2.0 workshop number 2 is still kinda low. I don't think its in danger of being cancelled, but if more attendees register then I'll be more confident we can have this thing.
The program is starting to take shape. The advanced concepts in the afternoon will definitely include:
How to combine quantitative whole protein and quantitative PTM (probably phosphoproteomics) into a single report AND
How to combine the results of multiple TMT and iTRAQ experiments.
We are still taking suggestions for other concepts to cover!
If you want to come, please register at this link: https://www.surveymonkey.com/r/?sm=PenWchsGrQuQZQmLH6odMzQoGuh4cDU%2fWF5ilEAy6oI%3d
15 Ekim 2015 Perşembe
MaxQuant summer school 2015 videos are all up!
It looks like all the MaxQuant videos are now available on Youtube:
These are the ones I've been able to find so far:
Basics 1 and 2
Protein quantification
Quantitative proteomics
From Discovery to Targeted Quan (with Brendan from Skyline)
Moving MS based quan to the clinic!
Label free quan
Recent developments in the Orbitrap (by Dr. Makarov!!!!!!)
Lysine acetylomics
An application interface for all those funny MS file types
All of our friends on the bioinformatics side of the proteomics world have been throwing out all these funny letters for years. They tend to start with an "m" and end with an "l" and have something random in the middle. mZmL, mzXmL, mzTab (no L! cheater!), mzIdentmL, and on and on. On cursory examination these are all attempts to store our data with better efficiency without the loss of data that we see when converting our data to MGF (where we lose almost all of our MS1 data!)
Problem is, that some of us have used these things. One or the other and the public repositories may have cool data hidden in one of these formats.
This new program (definitely meant for the bioinformaticians out there who can code and stuff!) is called ms-data-core-api. It is an Application Programming Interface that should take care of all these formats for you. Adding this to your programs will allow you to pull data in from any of these sources and read the data in a unifying format so you aren't all jumbled in your downstream processing.
You can read about it at BioCode's notes here.
And it can be downloaded at GitHub here.
14 Ekim 2015 Çarşamba
Panorama AutoQC?
I love webinars! In order of importance, this is what the internet has given my life:
1) Elvis Pugsley
(proof that I'm not the weirdest person in the world? or maybe just hilarious!!!)
2) Webinars! The ability to learn from lectures from wherever I happen to be. Now, webinars come in different levels of quality and topic interest but when I get and email from the MacCoss lab about a webinar I'm going to check it out.
And if it is on something I've never heard of (Panorama?) and it talks about how I can "AutoQC" my instruments (y'all might have realized I'm kind of a dork for quality control!) then I'm gonna sign up for it.
You can do the same at this link. Its next Tuesday (10/20/15) and starts at 11am Eastern.
SUMO E2 ligase is necessary for RAS/RAF oncogenesis
Just when you think a story can't get any more complex, biology up and surprises you!
This paper isn't brand new, but I don't check PNAS as often as some of the other purely -omics oriented journals. The paper in question is from Bing Yu et al., and is available here and is open access.
Its a cool story, too. There are really no targeted chemotherapies out there that will work on KRAS specific cancers. The function of the various GTPases and their pathways are complicated and convoluted. When they are working right they are supposed to function in signaling by GTP to GDP conversions. This communication system is so critical to normal cell functioning that disregulating of these proteins has the nasty outcomes of the cell dying or becoming a cancer cell. As in any biological system, its certainly more complex than this, because years of work with these things comes up with a whole lot more info and no clear simple answers.
This is where we come in. Turns out that this group did a big shRNA screen (this is where you transfect cells with a great big mixture of Single Hairpin RNA that knocks out RNA production (and therefore protein production) on a huge scale. The readout is typically a phenotype. In this case, I'm assuming what they did (I'm sure its in the paper. not my area of expertise.) was see what cells did or did not become cancerous and then go back and figure out what gene they knocked out.
Of the many observations they came with 2 ligases that are the only ones known to be involved in the SUMO E1 and E2 pathways (controlling the SUMO PTM, not sure if I have the nomenclature 100% correct here). Anyway...SUMOs are small proteins that are ligated to big proteins and modulate their function as post-translational modifications (PTMs). There are bunch of them and a bunch of pathways, but here you have two major regulators of SUMOylation (more info on this PTM here) that are somehow implicated in KRAS oncogenesis? Tell me more!
So, they go in and construct an RNA interference to directly deplete these SUMOylation ligases (the things that attach the SUMO PTMs) in some cells that are crazy KRAS cancer cells. Turns out that if you can't produce these proteins even a KRAS cancer cell gets subdued. Then they study it by labeling some of the cells with SILAC and repeating the knockdowns to try to figure out the mechanism by which all this is happening and come up with a group of proteins they call KASPs which is short for KRAS Associated SUMOylated Proteins that are involved in this mechanism.
To sum up: We start with a common cancer mutation we don't have drugs for and we figure out a protein, not just that, a whole series of proteins that may be potential targets for treatment when someone has this type of cancer. Inhibiting these proteins and maybe you have a new chemotherapy. And along the way, we learn an entirely new biological modulation pathway?
I highly recommend picking this one up. Its nice to see what we do fitting seamlessly into a biological study alongside the cutting edge tools the molecular biologists are using these days!
12 Ekim 2015 Pazartesi
Alzheimer's proteomics by high resolution differential analysis!
Alzheimer's disease is some terrifying stuff. Fortunately, however, it is a disease that appears to be amenable to study with the advanced tools we have these days! Pull a Google Scholar search for "Alzheimer's disease proteomics" and you'll find a load of great studies that show that protein mass spectrometry may be exactly the way that we need to approach this for early disease diagnosis, stage monitoring, and hopefully!!!! for finding the upstream stuff so we can fix it.
Point in case: This open access study from groups at several institutions (hey! my good friend Katie is an author!) who used a combination of differential proteomic analysis and targeted MS/MS to profile disease progression. For the initial analysis they use simply MS1 high resolution monitoring and compare the peak profiles between the CSF samples of different stages of the disease. These differential lists provide them with ions to go after in their targeted assays! This is a pretty awesome application of a way a lot of us have thought of doing proteomics over the years...by just going after the stuff that is different!
How'd they find the stuff that was different? With Elucidator (and I'm not sure it exists for today's instruments...) but we have lots of tools we could use for things like this, like SIEVE and OpenMS.
Big highlights of this assay? Seeing biomarkers without any level of antibody-based enrichment or pulldown. Just finding them by high resolution differentials!
Kaydol:
Yorumlar (Atom)
Popular Posts
-
A recent paper in PNAS makes the statement in the title "Protein Carbamylation is a Hallmark of Aging. You can find it here . They find...