5 Aralık 2015 Cumartesi
Confirmation of NIA standards for Alzheimer's disease via protein biomarkers
So, I've read 2 biology sciency things today. In both cases, the scientific method was at work (YAY!). Researchers were looking as published results and in the first case (the tardigrade genome I mentioned earlier in the week) striking problems were found with the data.
The second paper is more positive for the studies pre-dating it! In this paper from Huded et al., some researchers in India decided to test the National Institute on Aging's criteria for Alzheimer's diagnosis and progression. This requires removal of cerebral spinal fluid (CSF) and testing for a number of known protein biomarkers. Quantification reveals presence and severity of the disease.
Now, there hasn't just been one test on these biomarkers, there have been tons of them. So it would be super weird if they didn't check out properly (or you could blame it on the ELISA assays they were using). But, hey! sometimes you want to verify it yourself, especially if it requires extracting fluid from someone's nervous system!! On diseases that are this nefarious, every data point is going to help. Lets get early detection and drugs on the market, STAT!
4 Aralık 2015 Cuma
A team at UVA decided to rewrite the textbook on antibody profiling.
This is such a great paper! AND its Open Access. Several people who occasionally read my ramblings here who need to see this right now are about to get this link emailed directly to them! You're welcome!
The paper is from Lichao Zhang et al., and some guy named Don Hunt was apparently involved which might explain some things about it.
When I visit people who profile antibodies, they are doing 2 things. First they are getting intact masses on the antibody. In big facilities, maybe its a whole group of people figuring out what intact protein masses are there. The second thing is digesting with trypsin and peptide mapping. Between the two groups they pretty much figure out what they're looking at. Groups that use multiple enzymes get better coverage, but you're looking at a ton of runs.
This approach? Kind of a lower-middle down approach with just enough awesome tweaks to maybe get the whole antibody figured out in one shot!
They start with the whole antibody and then they reduce and alkylate it (more on that in a minute). Then they run it through or over an immobilized enzyme I've never ever heard of, aspergillopepsin I, which instantly cuts the antibody to pieces around 3-9 kDa long. See? Lower-middle-down! What else would you call it?
What else would you call peptides that are 3-9kDa long? Perfect for ETD! In this case they used an LTQ Orbitrap Velos with ETD. And these perfectly-sized fragments give off amazing levels of coverage. They process everything with ProsightPC BioMarker search functions.
Okay. Neat, right? But it gets better.
The digestion occurs with a bioreactor. The antibody goes in and comes out digested...and the reaction quenched. Want bigger fragments? Increase the flowrate. Smaller? Decrease it.
One last thing. They alkylate the cysteines with a new reagent. Its called NAEM
Not only does it alkylate in 10 minutes, but it also puts a positive charge on the cysteines which aids in fragmentation.
How's it work out? Absolutely ridiculous levels of coverage of these huge and hugely important proteins and their PTMs in record instrument time!
2 Aralık 2015 Çarşamba
What the heck is this metformin stuff, and how is it slowing aging?
So I'm sitting here watching the Q Exactive blow away yet another triple quad (the Q E is like "what's a matrix effect? never heard of it...") in terms of small molecule sensitivity and I have some time to browse Twitter and I see this article from June! What the
Human beings are being given an Anti-aging drug? Sign me up! Turns out you have to be suffering from one of three conditions to be eligible - cancer, heart disease, or cognitive impairment. You might argue I qualify under the third condition, but I think they mean decline from baseline and I've always been this way. The drug they are testing is metformin.
Next question is the title of this post. What does this stuff do?
Well, Chengkai Dai says its something like this (in this here paper):
In this paper he argues that HSF1 is repressed by nutrient stress and/or metformin and induces "proteomic chaos" and this messes up tumors. Which sounds pretty awesome. But what does this have to do with aging?
Time to turn off the "since 2015" filter on Google Scholar and travel far, far back in time!
So, this group dosed worms with the drug and did 2D-DIGE and peptide mass fingerprinting to come up with the solution that its all about reactive oxygen species and peroxiredoxin.
Which sounds an awful lot like what the resveratrol people have been thinking their drug does...either way this is super cool.
1 Aralık 2015 Salı
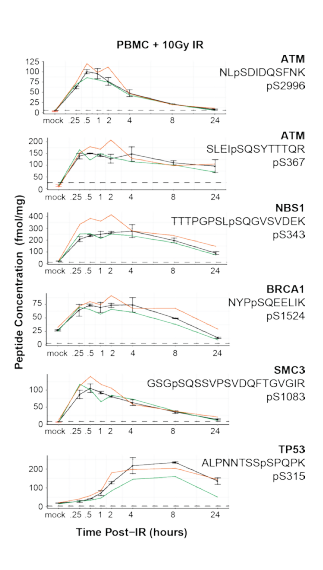
Can phospho- protein profiling be highly reproducible?
DNA damage response proceeds along a tightly controlled phosphorylation cascade. The main operators are very well studied and predictable enough that immuno assays are used in the clinic for these phosphorylation sites.
Could you quantify the entire cascade with a single LC-MS run? And could you do it with a high level of reproducibility? Sure looks like it!
In this paper from Jacob Kennedy et al., out of the Fred Hutch, they use a single step IMAC pull-down followed by MRMs and the data looks fantastic. (Max CV on phosphos of ~16%?!?!)
Do 150 Western blots? Or monitor all of this pathway in a single run? Makes the mass spec seem like a pretty cheap option, right? Now, being the resolution snob that I am, I would like to point out that the relatively small number of targets here, this is something that could easily be adapted to a Q Exactive. Again, the data looks seriously fantastic here, but if I was to improve this assay in any way I'd want to see my fragment ions in PRM +/- 1ppm.
Could you quantify the entire cascade with a single LC-MS run? And could you do it with a high level of reproducibility? Sure looks like it!
In this paper from Jacob Kennedy et al., out of the Fred Hutch, they use a single step IMAC pull-down followed by MRMs and the data looks fantastic. (Max CV on phosphos of ~16%?!?!)
Do 150 Western blots? Or monitor all of this pathway in a single run? Makes the mass spec seem like a pretty cheap option, right? Now, being the resolution snob that I am, I would like to point out that the relatively small number of targets here, this is something that could easily be adapted to a Q Exactive. Again, the data looks seriously fantastic here, but if I was to improve this assay in any way I'd want to see my fragment ions in PRM +/- 1ppm.
Tardigrade genome is done!
How on earth do you guys do proteomics on unsequenced organisms? I know there's BICEPS, which relies on genomes from similar organisms and error tolerant searches, but...besides that?
Okay, here are some good examples. They finally did the tardigrade genome, and its reeeeaaaalllly weird. But if the genome is only just now done, how did:
These groups do the proteomics studies in 2010 and 2011?
Well, the 2010 group did 2D-gels and compare their IDs against the current known protein sequences and the group in 2011 focuses primarily on highly conserved heat shock proteins.
So, you do what you can with the proteins that are in the databases. Or you study something that doesn't change from organism to organism.
Why is this cool, other than the fact that tardigrades are frickin awesome? Cause this is a perfect example of a great meta-analysis project. Google Scholar pulls up 5 studies that appear to be at least partial proteomic analyses of these ridiculously cool organisms. Every single one of them was performed with imperfect genomic or protein databases. If I was looking to write a nice and reasonably high impact paper this weekend, I'd be downloading this genome here and seeing how many of these papers submitted data to Tranche and PRIDE that I can freely download and meta-analyze. Then maybe we'd know how these weird, indestructible things tick...or dance...or swim....
UPDATE 12/5/15: Ummm...okay, so maybe hold off on that meta-analysis... the work of this study above has been an explosion in the genomics community. It appears there might have been some errors. I LOVE SEEING SCIENCE IN ACTION!!! Maybe all this is real, and maybe not, but either way we'll be further ahead! Discussion on the controversy here.
29 Kasım 2015 Pazar
Proteomics in forensics!
Whoa!!
Despite what crime dramas might lead us to believe, forensics technologies still aren't perfect. A big hangup of the DNA evidence is that we have the same copy of DNA in every cell, so telling where the tissue samples came from is difficult/impossible.
Sounds like a job for proteomics!
In this cool paper from Sascha Dammeier et al., out of the Kohlbacher lab these researchers investigate using a proteomics approach to show that shotgun proteomics runs can tell you what tissue some evidence comes from. When your Materials and Methods section includes bullets and evidence bags, you've entered into interesting proteomics territory!
To make it even more interesting(!!) they did the proof-of-principle stuff on a cow organs subjected to blunt trama and then(!!!) they participated in a real crime investigation!!!
Okay, get this. A bullet passing through and organ carries enough protein for an Orbitrap XL to get a proteome signature good identify the organ!
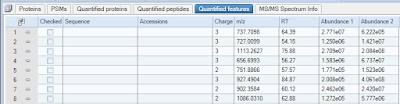
Label free proteomics in Proteome Discoverer 2.0 is GO!
Got Proteome Discoverer 2.0?
Want to get a free upgrade that gives you awesome levels of label free proteomics capabilities?
I mentioned this before, but I just installed the nodes that are listed here on a fresh install of PD 2.0 and ran it and I'm blown away. These are really really super nice!
My recommendations (cause I don't know what I did wrong before):
1) Read the instructions
2) Download the nodes AND the processing and consensus workflows
3) Don't use a small file to test. Percolator needs to run for this to work. In PD 2.0 if you have less than 200 peptides going into Percolator it just turns off. Then the nodes can't work.
4) Revel in your new capabilities!!!!
(Look at this!!! I can find stuff in my runs that were not identified, but that were differentially (sp?) regulated in my two samples!!!! MAGIC!)
5) Remember that this is a free second party software. There is a nice mailing group you can sign up to for advice and news about the nodes. Your instrument vendor probably can't help you with these.
6) Do awesome label free proteomics!!!
Kaydol:
Yorumlar (Atom)
Popular Posts
-
A recent paper in PNAS makes the statement in the title "Protein Carbamylation is a Hallmark of Aging. You can find it here . They find...